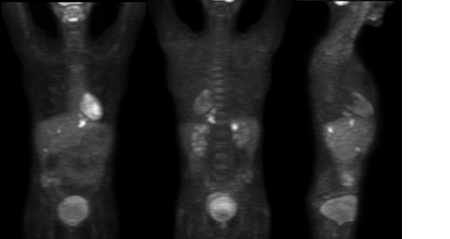

Anterior, posterior, and lateral projection images
View main image(pt) in a separate image viewer
View second image(pt). Anterior projection image, emission slices, and transmission slices.
View third image(pt). Coronal images
View fourth image(pt). Ultrasound images
Full history/Diagnosis is available below
Subsequent ultrasonography (image 4) shows an esophageal/proximal stomach mass, correlating with the known tumor. There also are at least four hypoechoic foci consistent with metastatic disease. Another hypoechoic lesion in the region of the gastrohepatic ligament may also represent metastasis.
Squamous cell esophageal carcinoma has the widest geographic variation in incidence of all tumors, thought to be due to environmental factors. Twenty percent of all deaths in the northern provinces of China and up to 50% of registered tumors in the Transkei region of South Africa are of esophageal squamous cell carcinoma origin. There is a 6.2-fold increased risk in patients who smoke more than 25 cigarettes per day. There is a 3.5-fold increased risk in patients who drink heavily. There is a 44-fold increased risk in patients who both smoke more than 30 g of tobacco and drink more than 120 g of alcohol per day. It is approximately 5 times more common in blacks than whites in the United States. Approximately 50% of esophageal squamous cell carcinomas occur in the middle esophagus, 33% in the lower esophagus and 17% in the upper esophagus (Gore).
Adenocarcinoma of the esophagus is nearly always associated with Barrett’s esophagus. It is a disease of white men, being 7 times more common in men than women and 4 times more common in whites than blacks. In some series, esophageal adenocarcinoma is more common than squamous cell carcinoma in white men in the United States. There is progressive columnar metaplasia in Barrett’s esophagus, which may then proceed to varying grades of dysplasia and, finally, to carcinoma. Most esophageal adenocarcinomas (almost 90%) occur in the distal esophagus (Gore).
The esophagus lacks a serosal surface that may otherwise impede the local spread of tumor. Mucosal lymphatics drain into a submucosal plexus that, in turn, drains into five channels in the muscularis. “Jump” lymph node metastases involving nodes in the mediastinum or neck are common. “Lymphatics draining the esophagus tend to follow arteries and mainly course longitudinally eventually draining into internal jugular, cervical, supraclavicular, paratracheal, hilar, subcardinal, paraesophageal, para-aortic, pericardial, left gastric, and celiac lymph node chains. Although the nodes involved by tumor are usually at the same level as the primary neoplasm, squamous cell carcinoma not uncommonly skips sets of lymph nodes at one level and invades more distal nodes. For example, lymph nodes in the gastrohepatic and hepatoduodenal ligaments drain the lower one third of the esophagus yet may be involved in 44% of middle third lesions, and up to 10% of upper third tumors” (Gore). Hematogenous metastases (to lung and liver) are commonly seen in advanced disease.
In endemic regions, screening techniques includes swallowing an abrasive balloon that is then inflated and pulled back through the esophagus. The retrieved material is then evaluated cytologically. Such techniques are roughly 80% accurate. In non-endemic areas, screening is not warranted, but patients who are at high risk or who have Barrett’s esophagus may be monitored. Patients with Barrett’s esophagus should be placed on antacid/antireflux measures. This has not been shown to slow development of dysplasia or cancer, but the reduction in inflammation makes interpretation of biopsy samples for dysplasia more accurate. Carcinoembryonic antigen (CEA) level preoperatively provides no prognostic information (unlike the case for colon carcinoma). Postoperatively, CEA levels can be used to help assess for recurrence. It has a “sensitivity of 55%, specificity of 90%, and positive predictive value of 91% in patients with recurrent esophageal cancer” (Gore).
The first step after diagnosis is accurate staging. Stages I and IIA are potentially curable with surgery. Some esophageal carcinomas are successfully treated with radiation therapy. Generally, radiation therapy is reserved for patients who are deemed surgically unresectable. There are, however, no randomized trials comparing surgical treatment with radiation therapy for esophageal carcinoma as of 1997 (Thomas). The TNM staging system is as follows:
Primary Tumor (T):
Tx: Primary tumor cannot be assessed
T0: No evidence of primary tumor
Tis: Carcinoma in situ
T1: Tumor invades lamina propria or submucosa
T2: Tumor invades muscularis propria
T3: Tumor invades adventitia
T4: Tumor invades adjacent structures
Regional lymph nodes (N):
Nx: Regional lymph nodes cannot be assessed
N0: No regional lymph node metastases
N1: Distant metastases
Stage grouping:
Stage 0: Tis N0 M0
Stage I: T1 N0 M0
Stage IIA: T2 N0 M0
T3 N0 M0
Stage IIB: T1 N1 M0
T2 N1 M0
Stage III: T3 N1 M0
T4 Any N M0
Stage IV: Any T Any N M1
Radiographic staging is oriented toward determination of tumor resectability. Endoscopy and standard radiographic techniques (barium swallow, plain film and CT) provide limited information regarding resectability. Plain film only rarely will show a mass effect or invasion of the mediastinum. Barium swallow and endoscopy can show the intraluminal extent of disease but do not give an indication of extent of invasion or lymph node metastasis. Computed tomography and MRI have been used with limited success. Modified TNM staging systems have been used for computed tomographic evaluation. The important CT criteria include “(1) the extent of involvement of the esophageal wall by tumor, (2) tumor invasion of the periesophageal fat and adjacent structures, and (3) metastases to regional nodes or distant organs” (Saunders).
CT is moderately useful for evaluation of the primary tumor. Some reports indicate that MRI is more accurate at defining tissue planes and assessing for invasion. However, both modalities show poor accuracy in evaluating lymph node disease. This limitation has also been shown in bronchogenic carcinoma, where CT evaluation of the mediastinum shows a sensitivity and specificity for detection of lymph node involvement in the mid 60-percent range. This is a serious drawback in esophageal carcinomas where most patients have lymph node disease at presentation. In the past, CT scan had a low accuracy with high specificity. Recent reports show a low specificity with many false-positives for lymph node involvement. The overall accuracy of CT scan in esophageal cancer is approximately 67%” (Krasna). Minimally invasive surgery may also be utilized for staging. Thoracoscopic and laparascopic lymph node staging were shown in a study by Krasna to be more accurate than staging with CT or MRI.
The use of FDG-PET for staging of esophageal carcinoma is still considered investigational by Medicare, in the United States. As of July, 1999, there are five Medicare-approved indications for use of body PET. These are: (1) evaluation of a solitary pulmonary nodule, (2) mediastinal staging of bronchogenic carcinoma, (3) suspected recurrent or metastatic melanoma, (4) history of treated colorectal carcinoma with a currently rising CEA level, and (5) staging/restaging of lymphoma. In bronchogenic carcinoma, PET has clearly been shown to improve staging accuracy over CT in numerous studies. With CT, the sensitivity and specificity for evaluation of mediastinal lymph nodes is in the mid-60-percent range. With PET, in areas not endemic for granulomatous infections, the sensitivity and specificity are in the mid-90-percent range. In areas endemic for granulomatous infections (for example, in the Ohio and Mississippi river valleys where histoplasmosis is endemic), the specificity drops modestly to the high-80-percent range.
Although PET is still considered investigational (by Medicare) for use with esophageal carcinoma and a number of other tumors, early reports (Luketich) and considerable experience at our institution indicate tremendous promise for PET in staging esophageal carcinoma. Cardiothoracic surgeons at Barnes-Jewish Hospital routinely use PET, in combination with CT, to stage esophageal carcinoma. This case highlights the usefulness of PET for accurate staging. The liver metastases were not apparent on the outside CT, but were picked up by PET (and subsequently ultrasonography), changing the stage. It is our experience that this, as in the case with bronchogenic carcinoma, is a common occurrence.
References:
Flanagan FL, et al. Staging of esophageal cancer with 18F-fluorodeoxyglucose positron emission tomography. AJR. 1684, 417-424. 1997
Gore R. Esophageal cancer. Rad Clin N America. 35, 243-294. 1997.
Krasna M. Advances in staging of esophageal carcinoma. Chest. 113, 107s-310s. 1998.
Luketich J, et al. Future directions in esophageal cancer. Intern symp on thoracic malign. Chest 113, 120s-122s. 1998.
Patti M, et al. Surgery of the esophagus. Surgical clinics of N America. 77, 959-968. 1997.
Saunders HS, Wolfman N and Ott D. Esophageal cancer, radiologic staging. Rad clin N America. 35, 281-292. 1998.
Thomas C. Biology of esophageal cancer and the role of combined modality therapy. Surgical clinics of N America. 77, 1139-1167. 1997.
References and General Discussion of PET Tumor Imaging Studies (Anatomic field:Gasterointestinal System, Category:Neoplasm, Neoplastic-like condition)
Return to the Teaching File home page.