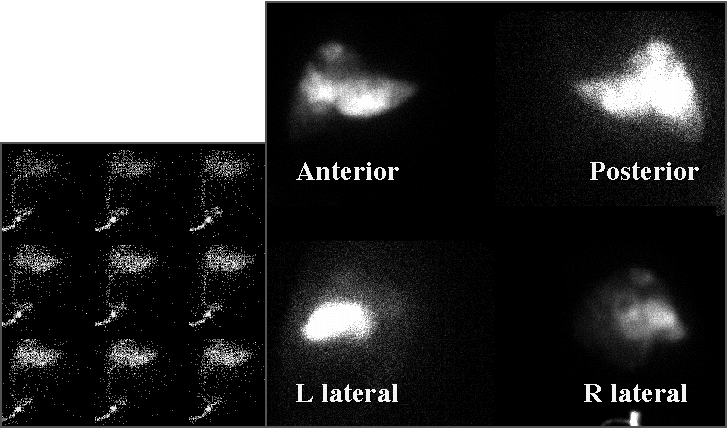

Hepatic perfusion scintigraphy (sequential images during injection, and static images)
View main image(hs) in a separate image viewer
View second image(ct). Computed tomography of liver
Full history/Diagnosis is available below
- No extrahepatic perfusion is seen.
2. Computed tomography (initial):
- S/P partial colectomy.
- Multiple low attenuation liver lesions, consistent with liver metastases.
3. Computed tomography (3.5 months later):
- S/P partial colectomy and chemotherapy infusion pump placement.
- Liver metastases in anterior and posterior segments of the right lobe of the liver. All are reduced in size or no longer visible since introduction of chemotherapy.
4. Computed tomography (6 months later (not shown)):
- Continued decrease in size of liver metastases.
Survival in patients with untreated liver metastases from colorectal carcinoma is very poor (on the order of 1 to 22 months). If the metastases are limited in number and extent (generally one lobe), cure can be effected by surgical excision. Prognosis is considered best if there are fewer than four metastases and if surgical margins are > 1 cm. Five-year survival for patients who have had up to three liver metastases resected is approximately 25%.
If surgical resection is not an option, chemotherapy may be tried. However, systemic chemotherapy only yields a response in 10 to 30% of patients. Selective hepatic arterial chemotherapy with an agent that is metabolized quickly and that is delivered by a catheter in the hepatic artery offers the advantage of direct delivery of chemotherapy to the liver, allowing for higher doses than would be possible systemically (usually bone marrow or gut epithelial dose is a limiting factor in systemic chemotherapy). Hepatic arterial chemotherapy offers the additional advantage of preferential delivery to tumor over liver parenchyma due to the preferential blood supply of tumors by the hepatic artery. The mean tumor : nontumor ratio is approximately 3 to 1. Hepatic arterial chemotherapy has reported response rates of 34 to 72%. Four randomized trials failed to show a survival benefit with hepatic arterial chemotherapy over systemic chemotherapy (although 2 meta analyses do show a response rate advantage and a trend toward better survival). Two randomized trials show a survival advantage of hepatic arterial chemotherapy over patients treated with supportive care alone (Venook).
Hepatic arterial chemotherapy has been successfully used for tumors other than colorectal metastases. It has also been reported for use in adjuvant therapy for colorectal carcinoma after surgical resection of hepatic metastases (Nonami). The rationale for this being that liver recurrence is the main cause of death in this group of patients (approximately 60% will experience recurrence). Hepatic recurrence was shown to be significantly reduced with hepatic arterial chemotherapy. However, there is also a significant rate of pulmonary recurrences which is not addressed by hepatic arterial chemotherapy. It is hypothesized by Nonami that a combination of hepatic arterial and systemic adjuvant chemotherapy may maximize survival.
Initially, angiography is performed to assess celiac axis anatomy. Only 61% showed conventional anatomy in a study of 550 patients at UCSF (Venook). Exploratory laparotomy is performed next. The presence of non-resectable tumor is evaluated first. A cholecystectomy is performed. The catheter is then placed surgically. The success rate of the catheter placement is dependant on the surgeon s experience (many early problems were reported in the literature). Five-ml of florescein dye is injected and the liver, stomach and duodenum are visualized under a wood s lamp. An additional check for liver perfusion and extrahepatic misperfusion is made after surgery with a Tc-99m hepatic arterial perfusion scintigraphy examination. The tracer is injected slowly to avoid reflux out of the primary vessel due to high injection rate (a rapid bolus injection would risk such reflux and not give a realistic indication of the chemotherapeutic perfusion since the chemotherapeutic agent is infused very slowly - for example, 5ml over 3 hours each day for 14 days). The scintigraphic method is more sensitive for detection of subtle misperfusion than angiography. Stomach or duodenal ulceration may result if misperfusion occurs during chemotherapy administration. If ulceration occurs, methylene blue dye may be injected during endoscopic observation of the ulcer. If blue dye is observed, angiographic evaluation is undertaken to search for the offending vessel (which can commonly be occluded by interventional radiology).
Arteriovenous shunting through the liver allows increased chemotherapeutic agent to reach the systemic circulation and less is delivered to tumor. A small amount of shunting (1 to 7%) is common. However, it may reach 40%. This will manifest as lung uptake.
Hepatic arterial perfusion scintigraphy can also be used preoperatively to assess for metastatic liver lesions. The 3 to 1 tumor to liver uptake ratio allows for visualization of metastatic lesions with higher sensitivity than scintigraphy with sulfur colloid or computed tomography.
References:
Carter R, et al. A prospective study of six methods for detection of hepatic colorectal metastases. Ann R Coll Surg Engl. 78, 27-30. 1996.
Cohen A, et al. Is intra-arterial chemotherapy worthwhile in the treatment of patients with unresectable hepatic colorectal cancer metastases? Eur J of Cancer. 32A, 2195-2205. 1996.
Nonami T et al. Regional adjuvant chemotherapy after partial hepatectomy for metastatic colorectal carcinoma. Sem in Onc. 26; s6, 130-134. 1997.
Thrall J and Ziessman H. Nuclear Medicine, the requisites. 1994.
Venook A. Update on Hepatic Intraarterial chemotherapy. Onc. 11; 7, 947-957. 1997.
Yamamoto K and Tanaka Y. Radiofrequency capacitive hyperthermia for unresectable hepatic cancers. J Gastroent. 32, 361-366. 1997.
References and General Discussion of Hepatobiliary Scintigraphy (Anatomic field:Gasterointestinal System, Category:Neoplasm, Neoplastic-like condition)
Return to the Teaching File home page.