|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
An Imaging Pilot Study using In-111 DTPA-Folate to Determine the Ability of Folate
Conjugates to Target Folate Receptors in Ovarian Cancer
Principal Investigator:Barry A. Siegel, M.D.
|
|
|
|
|
|
|
For Further Information Contact:
|
|
|
Helen Kaemmerer or Jennifer Frye at
314-362-7026
|
|
|
|
|
|
|
|
|
|
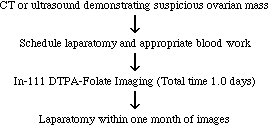
PURPOSE:This study is designed to determine whether an experimental radioactive drug In-111 DTPA
Folate will be taken up in ovarian tumors so that the tumors can be seen on pictures (images) obtained by
standard nuclear medicine imaging methods. This will be accomplished by comparing the results of the
nuclear medicine images with those of imaging tests that are currently used to diagnose ovarian tumors
(CT scanning and ultrasound scanning. The results will also be compared with findings at subsequent
surgery.
ELIGIBILITY CRITERIA:
|
|
|
|
|
|
1.
2.
|
|
Patients must be 18 years of age or older
Patients must have either:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
a.
b.
|
|
an ovarian mass suspected to represent ovarian cancer diagnosed by CT or ultrasound scanning
suspected recurrent ovarian cancer diagnosed by CT or ultrasound scanning
|
|
|
|
|
3.
4.
5.
6.
|
|
Patients must be scheduled or about to be scheduled for laparatomy
Patients cannot have received chemotherapy or radiation therapy in the past 3 months
Patients must have good kidney and liver function (as proven by blood testing, which is provided
free of charge to those who are eligible to participate)
Patients must be able to give informed consent
|
|
|
|
|
|
|
|
|
Those patients noteligible to participate:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1.
2.
3.
4.
5.
6.
7.
8.
9.
|
|
Patients not able to give informed consent
Patients who are pregnant or lactating
Patient with a history of heart trouble or a heart attack in the past 6 months
Patients with septicemia, severe infection, or acute hepatitis
Patients with known or suspected kidney disease
Patients taking folate-containing vitamins that cannot be suspended for 2 days prior to the study.
Patients taking drugs thatimpair renal function
Patients with inadequate bone marrow, renal or hepatic function
Patients with preexisting condition that prevents administration of large fluid volumes over a short
period of time
|
|
|
|
|
|
|
|
YOUR PARTICIPATION WOULD INVOLVE:
Approximately one day of your time. The first day is divided into two parts, initial images and delayed
images. Initial images take approximately 90 minutes. A second set of images is taken 4-6 hours after the
initial images (total imaging time for the second set of images is 60-90 minutes). There is no charge to you
or your insurance company for participating
|
|
|
|